Integrative study of marine microbial communities
Systems biology approach to the study of the most numerically abundant phototroph in the global oceans
Prior to my time as a graduate student I worked in the Chisholm Lab of Microbial Oceanography and Cross-Scale Systems Biology studying all things to related to a group of picocyanobacteria called Prochlorococcus. Members of this group are the numerically dominant phototrophs of the worlds oceans: at their most dense reaching upwards of 100,000 cells/mL of seawater and estimated to contribute to 5% of global photosynthesis. I was able to cut my teeth in microbiology working with these fastidious organisms in pure culture and in co-culture experiments exploring many aspects of their microbial ecology and photo- and thermal physiology.
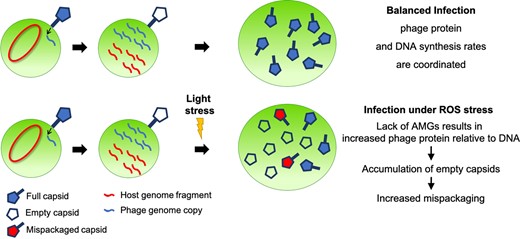
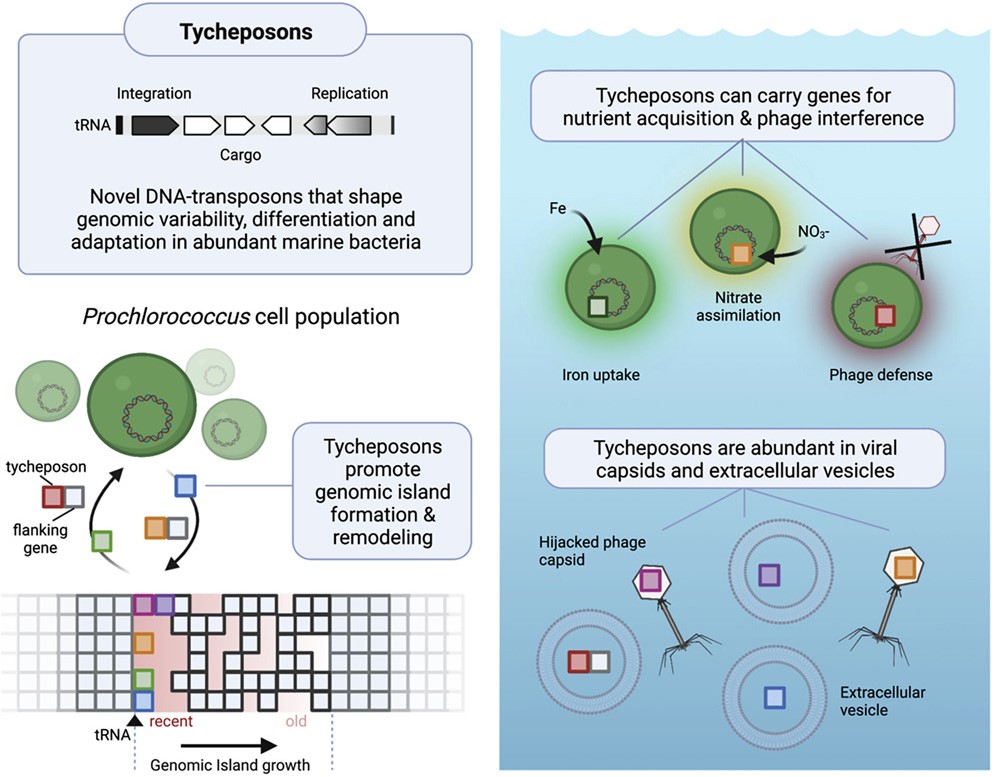
In terms of Prochlorococcus as a driver of genetic diversity, I first contributed to work describing cyanophage mispackaging of host genome, a possible mechanism of horizontal gene transfer in the global oceans. At higher light levels, such as in surface waters, we proposed that the rate of protein synthesis may outpace phage genome replication. In infected cells, this imbalance could increase the frequency with which phage capsids are mispackaged with fragments of host DNA instead of viral genomes. In separate work, we described tycheposons, a novel family of mobile genetic elements that drive genomic plasticity in Prochlorococcus and related marine microbes. These elements integrate at tRNA genes, carry adaptive cargo (such as nutrient assimilation genes), and can spread via extracellular membrane vesicles and phage particles, offering a new mechanism for horizontal gene transfer in the oceans.
I also contributed to work describing how metabolic products derived from Prochlorococcus might be trafficked through communities of microorganisms in excreted extracellular vesicles, documenting the rates of vesicle production across strains and abiotic gradients as well as describing the contents of vesicles and their association with the membranes of diverse marine bacteria. We notably detected vesicle-mediated interactions between Prochlorococcus and the numerically-dominant bacterioplankton of the world’s oceans, Pelagibacter ubique.


Another fundamental area we set out to explore in the Prochlorococcus system was ability of this group of organisms to survive periods of extended darkness. This was of interest given the high sensitivity of pure cultures to variation in light levels, yet the dynamic conditions of light availability in the global oceans due to intermittent mixing below the euphotic zone. In a pair of recent papers, we first described an as-yet undetermined epigenetic mechanism by which Prochlorococcus is able to heritably acquire dark tolerance. Next, we used transcriptomics to describe how metabolic coupling to “helper bacteria” in the ocean underlies this shift to dark tolerance. Prochlorococcus downregulated photosynthesis and upregulated respiration and biosynthesis concomitant with a co-occurring helper bacterium upregulating its consumption of organic acids, suggesting enhanced carbon exchange. These observations were supportive of the idea that overflow metabolism from marine heterotrophs sustains Prochlorococcus through dark stress long enough to enable its epigenetic adaptation.
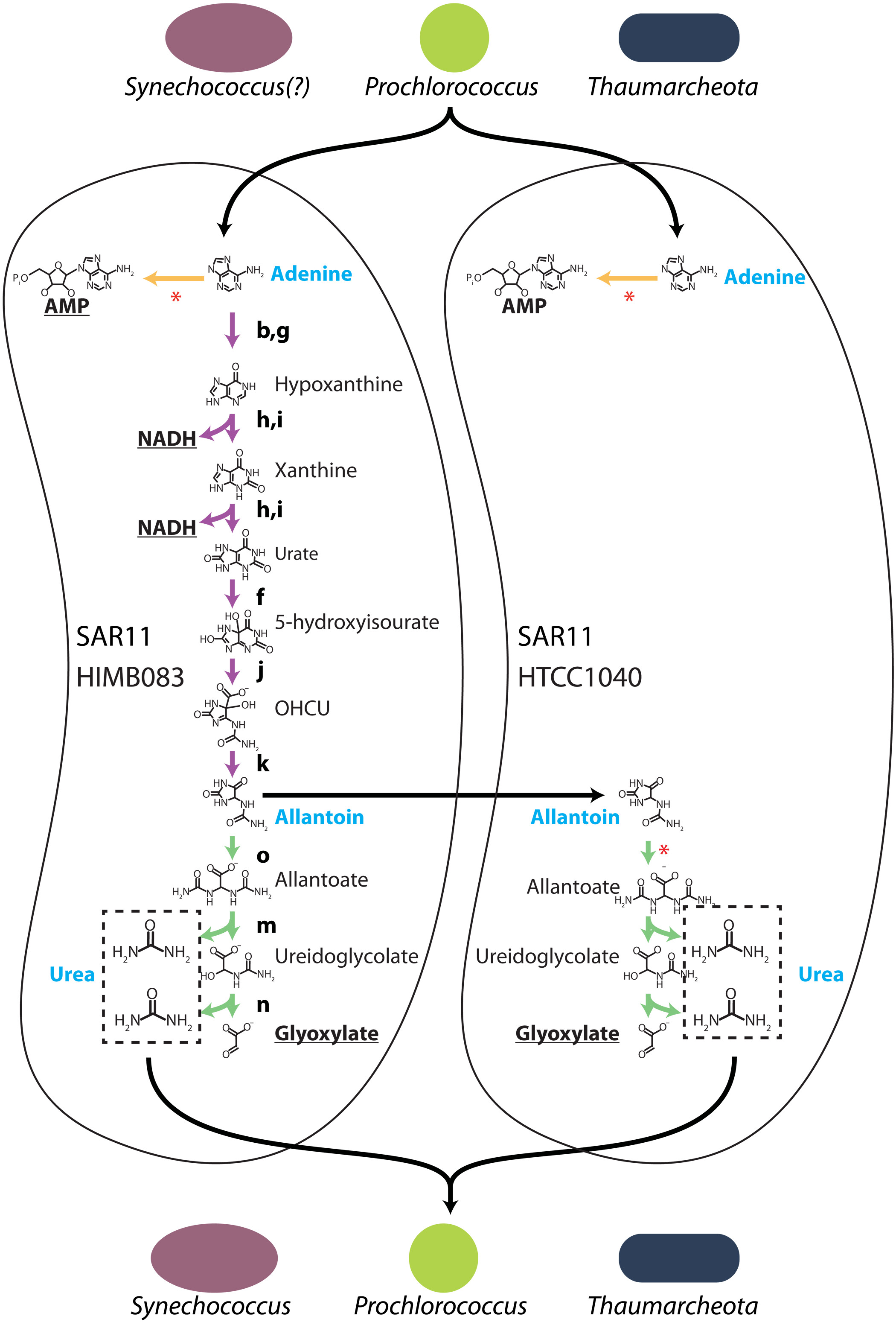
Lastly, I contributed to work linking Prochlorococcus exudates (purines and pyrimidines) and nitrogen specialization by members of Pelagibacter ubique in the global oceans. Prochlorococcus rhythmically exudes purines and pyrimidines, likely related to daily genome replication. Co-occurring heterotrophs appear to specialize in consuming these exudates either as generalists or purine/pyrimidine specialists, which supports elemental cycling and emerging cryptic nitrogen loops across ocean regions. While these hypotheses could be gleaned from sequencing data, we further utilized a pure culture of Pelagibacter ubique) and media amendments of adenine to demonstrate delayed DNA synthesis, supportive of the hypothesis that marine heterotrophs sync replication to the timing of photosynthate availability from phytoplankton like Prochlorococcus.
Associated references:
Coe, A., Mullet, J.I.,Vo, N.N., Berube, P.M., Anjur-Dietrich, M.I., and Salcedo, E., Parker, S.M., VonEmster, K., Bliem, C., Arellano, A.A., Castro, K.G., Becker, J.W., & Chisholm, S.W. (2025). A curated protein dataset for taxonomic classification of Prochlorococcus and Synechococcus in metagenomes. Scientific Data
Braakman, R., Satinsky, B., O’Keefe, T. J., Longnecker, K., Hogle, S.L., Becker, J. W., Dooley, K., Arellano A.A., Kido Soule, M.C., Kujawinski, E.B., & Chisholm, S. W. (2025). Global niche partitioning of purine and pyrimidine cross-feeding among ocean microbes. Science Advances.
Coe, A., Braakman, R., Biller, S.J., Arellano,A.A., Bliem,C., Vo,N.N., von Emster,K., Thomas, E., DeMers, M., Steglich, C., Huisman, J., & Chisholm, S.W. (2024). Emergence of metabolic coupling to the heterotroph Alteromonas promotes dark survival in Prochlorococcus. ISME Communications.
Becker, J. W., Pollak, S., Berta-Thompson, J. W., Becker, K. W., Braakman, R., Dooley, K. D., Hackl, T., Coe, A., Arellano, A.A., LeGault, K.N., Berube, P.M., Biller, S.J., Cubilloz-Ruiz, A.,Van Mooy, B.A.S., & Chisholm, S. W. (2024). Novel isolates expand the physiological diversity of Prochlorococcus and illuminate its macroevolution. mBio.
Biller, S.J., Coe, A., Arellano, A.A., Dooley, K., Gong, J., Yeager, E.A., Becker, J.W., & Chisholm, S.W. (2023). Environmental and taxonomic drivers of microbial vesicle production in marine ecosystems. Applied and Environmental Microbiology.
Hackl, T, Laurenceau, R, Ankenbrand, M.J., Bliem, C, Cariani, Z, Thomas, E, Dooley, K.D., Arellano, A.A., Hogle, S.L., Berube, P.M., Leventhal, G.E., Luo, E, Eppley, J, Zayed, A.A., Beaulaurier, J, Stephanauskas, R, Sullivan, M.B., Delong, E.F., Biller, S.J., & Chisholm, S.W. (2023). Novel integrative elements and genomic plasticity in ocean ecosystems. Cell.
Biller, S.J., Lundeen, R.A., Hmelo, L.R., Becker, K.W., Arellano, A.A., Dooley, K., Heal, K.R., Carlson, L.T., Van Mooy, B.A.S., Ingalls, A.E., & Chisholm, S.W. (2021). Prochlorococcus extracellular vesicles: molecular composition and adsorption to diverse microbes. Environmental Microbiology.
Coe, A., Biller, S.J., Thomas, E., Boulias, K., Bliem, C., Arellano, A.A., Dooley, K., Rasmussen, A.N., LeGault, K., O’Keefe, T.J., Stover, S., Greer, E.L., & Chisholm, S.W. (2021). Coping with darkness: The adaptive response of marine picocyanobacteria to repeated light energy deprivation. Limnology and Oceanography.
Laurenceau, R., Raho, N., Forget, M., Arellano, A. A., & Chisholm, S. W. (2021). Frequency of mispackaging of Prochlorococcus DNA by cyanophage. The ISME Journal.