publications
publications by categories in reversed chronological order. generated by jekyll-scholar.
2025
-
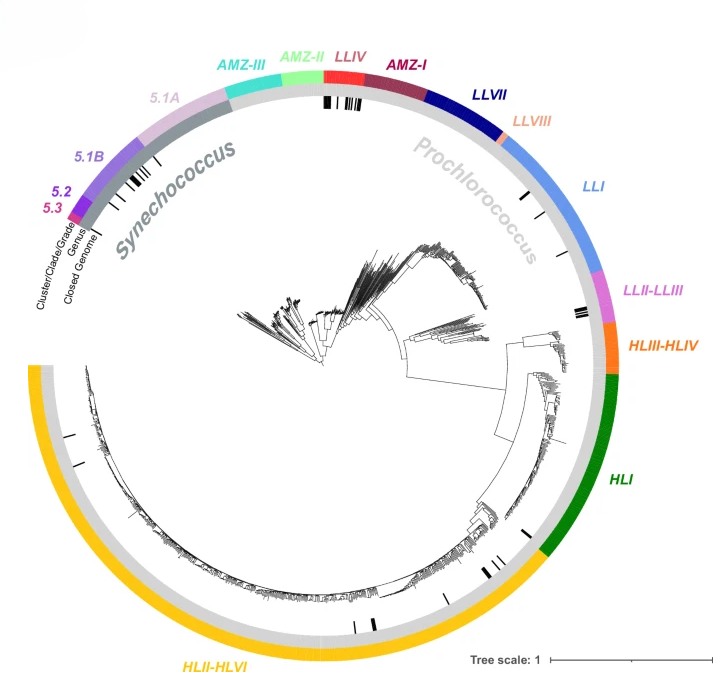 A curated protein dataset for taxonomic classification of Prochlorococcus and Synechococcus in metagenomesAllison Coe, James I Mullet, Nhi N Vo , and 8 more authorsScientific Data, Dec 2025
A curated protein dataset for taxonomic classification of Prochlorococcus and Synechococcus in metagenomesAllison Coe, James I Mullet, Nhi N Vo , and 8 more authorsScientific Data, Dec 2025Prochlorococcus and Synechococcus are abundant marine picocyanobacteria that contribute significantly to ocean primary production. Recent genome sequencing efforts, including those presented here, have yielded a large number of high-quality reference genomes, enabling the classification of these picocyanobacteria in marine metagenomic sequence data at high phylogenetic resolution. When combined with environmental data, these classifications can guide cluster/clade/grade assignments and offer insights into niche differentiation within these populations. Here we present ProSynTax, a curated protein sequence dataset and accompanying classification workflow aimed at enhancing the taxonomic resolution of Prochlorococcus and Synechococcus classification. ProSynTax includes proteins from 1,260 genomes of Prochlorococcus and Synechococcus, including single-amplified genomes, high-quality draft genomes, and newly closed genomes. Additionally, ProSynTax incorporates proteins from 41,753 genomes of marine heterotrophic bacteria, archaea, and viruses to assess microbial and viral communities surrounding Prochlorococcus and Synechococcus. This resource enables accurate classification of picocyanobacterial clusters/clades/grades in metagenomic data – even when present at 0.15% of reads for Prochlorococcus or 0.03% of reads for Synechococcus.
-
 Host-mediated niche construction of bacterial communities in an aquatic microecosystemAldo A Arellano, Journey L Prack, and Kerri L CoonThe ISME Journal, Oct 2025
Host-mediated niche construction of bacterial communities in an aquatic microecosystemAldo A Arellano, Journey L Prack, and Kerri L CoonThe ISME Journal, Oct 2025Microbes coordinate homeostasis in host-associated and environmental ecosystems alike, but the connectivity of these biomes is seldom considered. Hosts exert controls on the composition and function of their internally associated symbionts, but an underappreciated modality of microbiome curation is external to the host through changes to the environmental species pool from which they recruit microbial symbionts. Niche construction theory describes how organisms alter their environment and the selective landscape of their offspring and conspecifics. We hypothesize that host-driven manipulation of environmental microbial communities is an underexplored form of this concept. Using the pitcher plant mosquito (Wyeomyia smithii) as a model, we tested how hosts shape microbial communities across developmental stages and gradients of pre-existing community complexity. We report three lines of evidence supporting host-mediated niche construction, leveraging amplicon sequencing and microbiota manipulation experiments with germ-free (axenic) and selectively recolonized (gnotobiotic) mosquitoes. First, single female egg-laying assays showed repeatable adult inoculation of sterile water with beneficial bacteria capable of sustaining robust larval development. Second, increasing larval density in assays inoculated with complex, field-derived microbial communities selected for environmental and host-associated bacteria that correlated with increased larval fitness. Finally, exposing axenic larvae to mixtures of parentally and environmentally derived microbiota demonstrated that prior conditioning by conspecifics enhanced offspring fitness. Although the bacterial taxa associated with mosquito structuring varied, members of the Actinobacteriota and Acetobacteraceae were consistently associated with increased fitness. Overall, our results provide an example of host-mediated niche construction to favor environmental microbial communities that positively impact host fitness.
-
 Global niche partitioning of purine and pyrimidine cross-feeding among ocean microbesRogier Braakman, Brandon Satinsky, Tyler J O’Keefe , and 8 more authorsScience Advances, Jan 2025
Global niche partitioning of purine and pyrimidine cross-feeding among ocean microbesRogier Braakman, Brandon Satinsky, Tyler J O’Keefe , and 8 more authorsScience Advances, Jan 2025Cross-feeding involves microbes consuming exudates of other surrounding microbes, mediating elemental cycling. Characterizing the diversity of cross-feeding pathways in ocean microbes illuminates evolutionary forces driving self-organization of ocean ecosystems. Here, we uncover a purine and pyrimidine cross-feeding network in globally abundant groups. The cyanobacterium Prochlorococcus exudes both compound classes, which metabolic reconstructions suggest follows synchronous daily genome replication. Co-occurring heterotrophs differentiate into purine- and pyrimidine-using generalists or specialists that use compounds for different purposes. The most abundant heterotroph, SAR11, is a specialist that uses purines as sources of energy, carbon, and/or nitrogen, with subgroups differentiating along ocean-scale gradients in the supply of energy and nitrogen, in turn producing putative cryptic nitrogen cycles that link many microbes. Last, in an SAR11 subgroup that dominates where Prochlorococcus is abundant, adenine additions to cultures inhibit DNA synthesis, poising cells for replication. We argue that this subgroup uses inferred daily adenine pulses from Prochlorococcus to synchronize to the daily photosynthate supply from surrounding phytoplankton.
2024
-
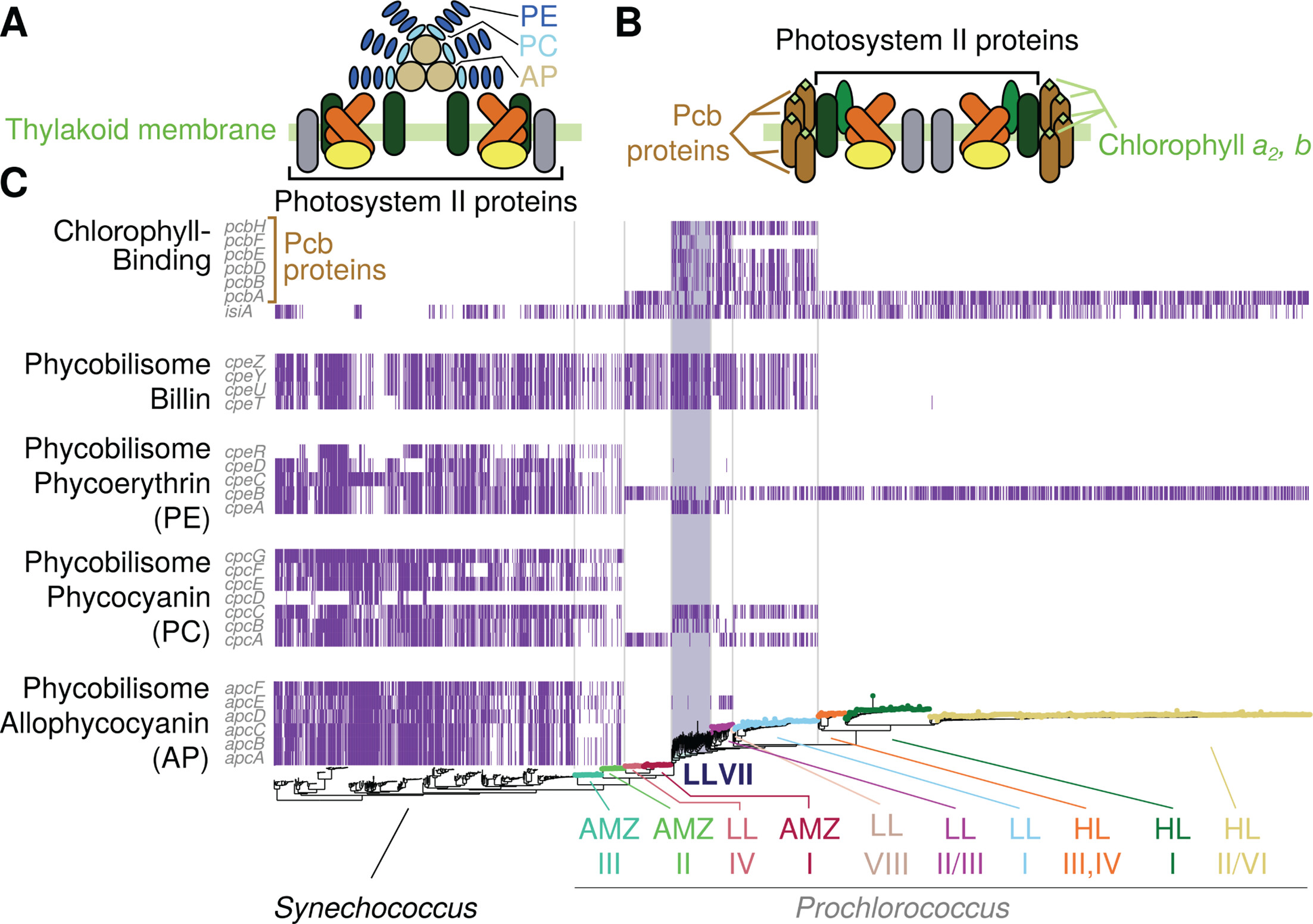 Novel isolates expand the physiological diversity of Prochlorococcus and illuminate its macroevolutionJamie W Becker, Shaul Pollak, Jessie W Berta-Thompson , and 8 more authorsMbio, Nov 2024
Novel isolates expand the physiological diversity of Prochlorococcus and illuminate its macroevolutionJamie W Becker, Shaul Pollak, Jessie W Berta-Thompson , and 8 more authorsMbio, Nov 2024Prochlorococcus is a diverse picocyanobacterial genus and the most abundant phototroph on Earth. Its photosynthetic diversity divides it into high-light (HL)- or low-light (LL)-adapted groups representing broad phylogenetic grades—each composed of several monophyletic clades. Here, we physiologically characterize four new Prochlorococcus strains isolated from below the deep chlorophyll maximum in the North Pacific Ocean. We combine these physiological properties with genomic analyses to explore the evolution of photosynthetic antennae and discuss potential macroevolutionary implications. The isolates belong to deeply branching low-light-adapted clades that have no other cultivated representatives and display some unusual characteristics. For example, despite its otherwise low-light-adapted physiological characteristics, strain MIT1223 has low chl b2 content similar to high-light-adapted strains. Isolate genomes revealed that each strain contains a unique arsenal of pigment biosynthesis and binding alleles that have been horizontally acquired, contributing to the observed physiological diversity. Comparative genomic analysis of all picocyanobacteria reveals that Pcb, the major pigment carrying protein in Prochlorococcus, greatly increased in copy number and diversity per genome along a branch that coincides with the loss of facultative particle attachment. Collectively, these observations support a recently developed macroevolutionary model, in which niche-constructing radiations allowed ancestral lineages of picocyanobacteria to transition from a particle-attached to planktonic lifestyle and broadly colonize the euphotic zone.
-
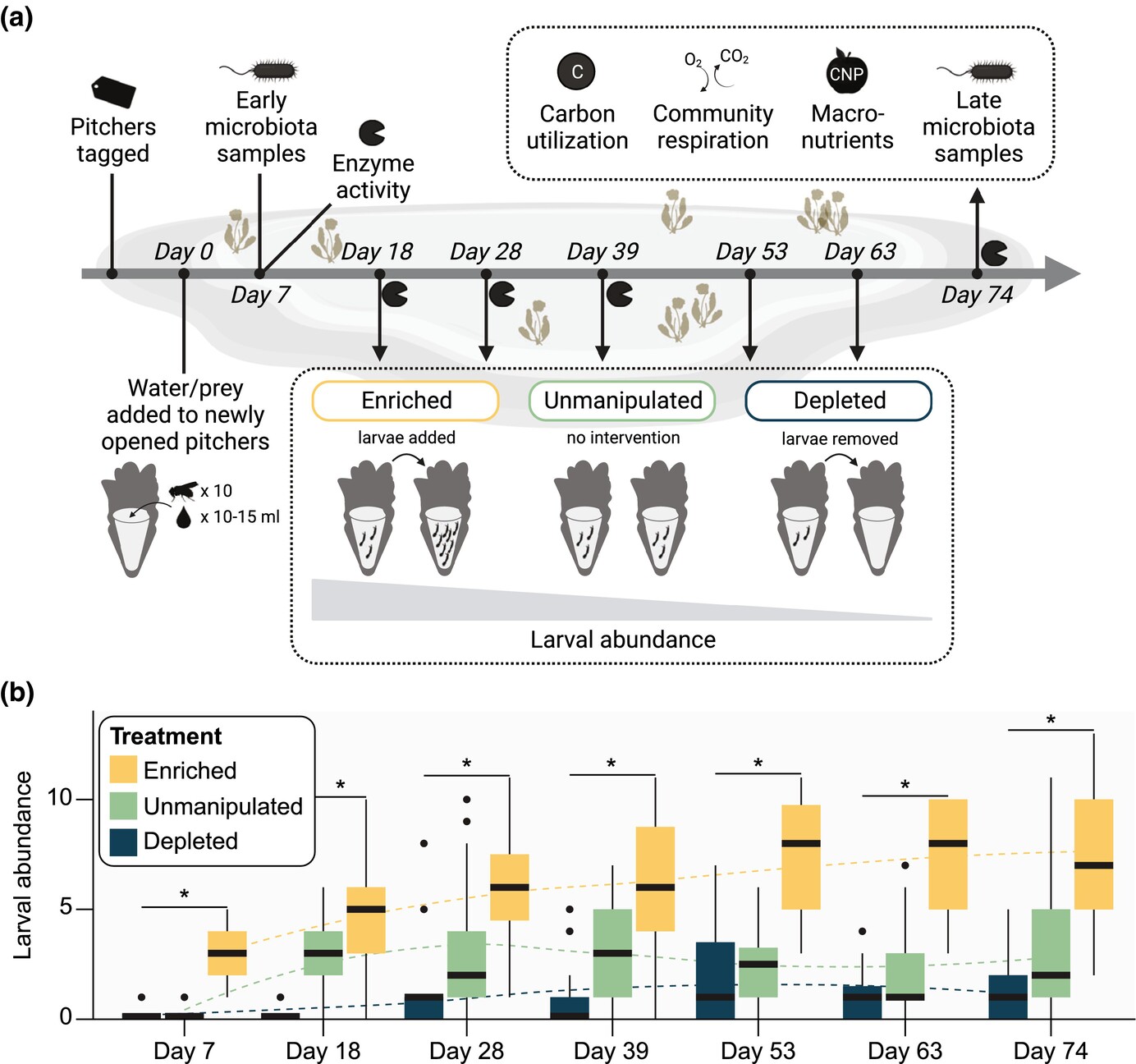 An inquiline mosquito modulates microbial diversity and function in an aquatic microecosystemAldo A Arellano, Erica B Young, and Kerri L CoonMolecular Ecology, Apr 2024
An inquiline mosquito modulates microbial diversity and function in an aquatic microecosystemAldo A Arellano, Erica B Young, and Kerri L CoonMolecular Ecology, Apr 2024Understanding microbial roles in ecosystem function requires integrating microscopic processes into food webs. The carnivorous pitcher plant, Sarracenia purpurea, offers a tractable study system where diverse food webs of macroinvertebrates and microbes facilitate digestion of captured insect prey, releasing nutrients supporting the food web and host plant. However, how interactions between these macroinvertebrate and microbial communities contribute to ecosystem functions remains unclear. We examined the role of the pitcher plant mosquito, Wyeomyia smithii, in top-down control of the composition and function of pitcher plant microbial communities. Mosquito larval abundance was enriched or depleted across a natural population of S. purpurea pitchers over a 74-day field experiment. Bacterial community composition and microbial community function were characterized by 16S rRNA amplicon sequencing and profiling of carbon substrate use, bulk metabolic rate, hydrolytic enzyme activity, and macronutrient pools. Bacterial communities changed from pitcher opening to maturation, but larvae exerted minor effects on high-level taxonomic composition. Higher larval abundance was associated with lower diversity communities with distinct functions and elevated nitrogen availability. Treatment-independent clustering also supported roles for larvae in curating pitcher microbial communities through shifts in community diversity and function. These results demonstrate top-down control of microbial functions in an aquatic microecosystem.
-
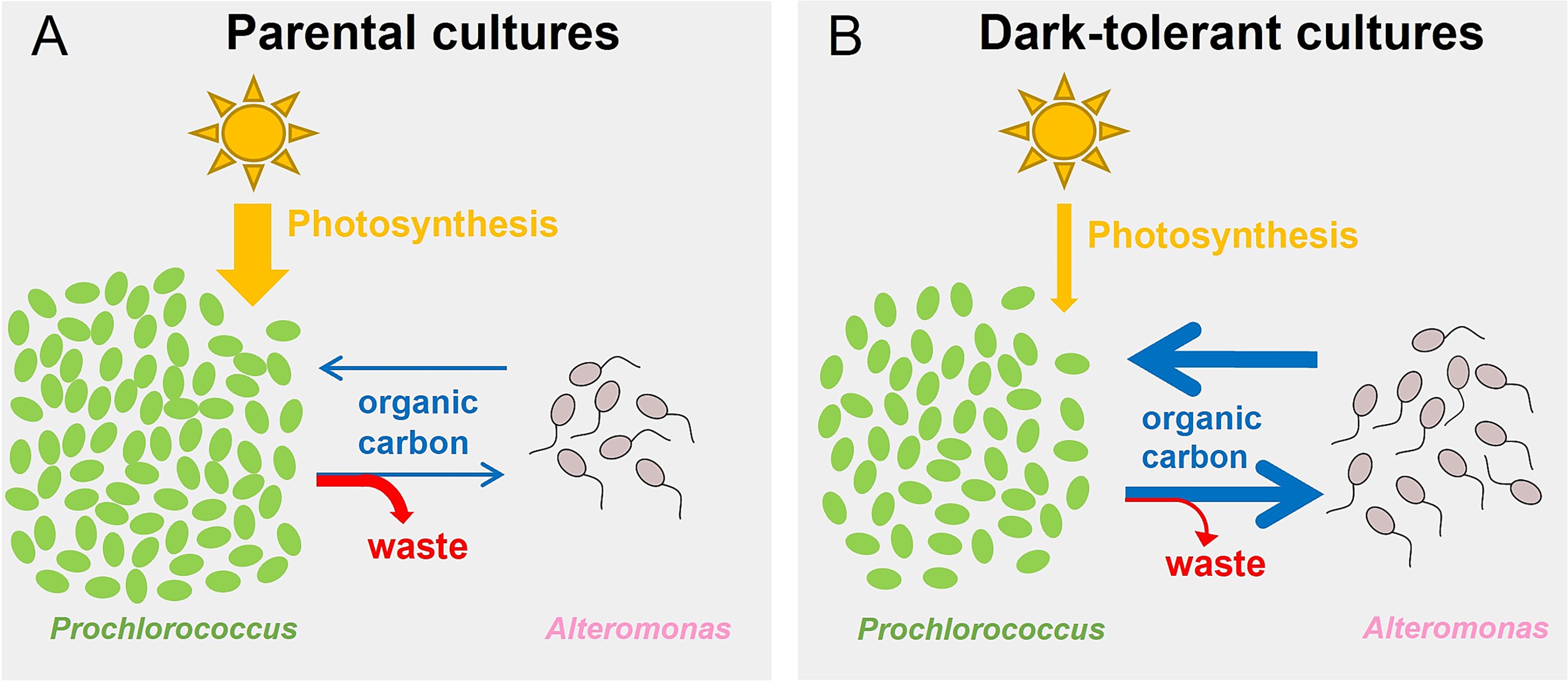 Emergence of metabolic coupling to the heterotroph Alteromonas promotes dark survival in ProchlorococcusAllison Coe, Rogier Braakman, Steven J Biller , and 8 more authorsISME communications, Jan 2024
Emergence of metabolic coupling to the heterotroph Alteromonas promotes dark survival in ProchlorococcusAllison Coe, Rogier Braakman, Steven J Biller , and 8 more authorsISME communications, Jan 2024Prochlorococcus is found throughout the euphotic zone in the oligotrophic open ocean. Deep mixing and sinking while attached to particles can, however, transport Prochlorococcus cells below this sunlit zone, depriving them of light for extended periods of time. Previous work has shown that Prochlorococcus by itself cannot survive extended periods of darkness. However, when co-cultured with a heterotrophic microbe and subjected to repeated periods of extended darkness, Prochlorococcus cells develop an epigenetically inherited dark-tolerant phenotype that can survive longer periods of darkness. Here we examine the metabolic and physiological changes underlying this adaptation using co-cultures of dark-tolerant and parental strains of Prochlorococcus, each grown with the heterotroph Alteromonas under diel light:dark conditions. The relative abundance of Alteromonas was higher in dark-tolerant than parental co-cultures, while dark-tolerant Prochlorococcus cells were larger, contained less chlorophyll, and were less synchronized to the light:dark cycle. Meta-transcriptome analysis revealed that dark-tolerant co-cultures undergo a joint change, in which Prochlorococcus undergoes a relative shift from photosynthesis to respiration, while Alteromonas shifts toward using more organic acids instead of sugars. Furthermore, the transcriptome data suggested enhanced biosynthesis of amino acids and purines in dark-tolerant Prochlorococcus and enhanced degradation of these compounds in Alteromonas. Collectively, our results demonstrate that dark adaptation involves a strengthening of the metabolic coupling between Prochlorococcus and Alteromonas, presumably mediated by an enhanced, and compositionally modified, carbon exchange between the two species.
2023
-
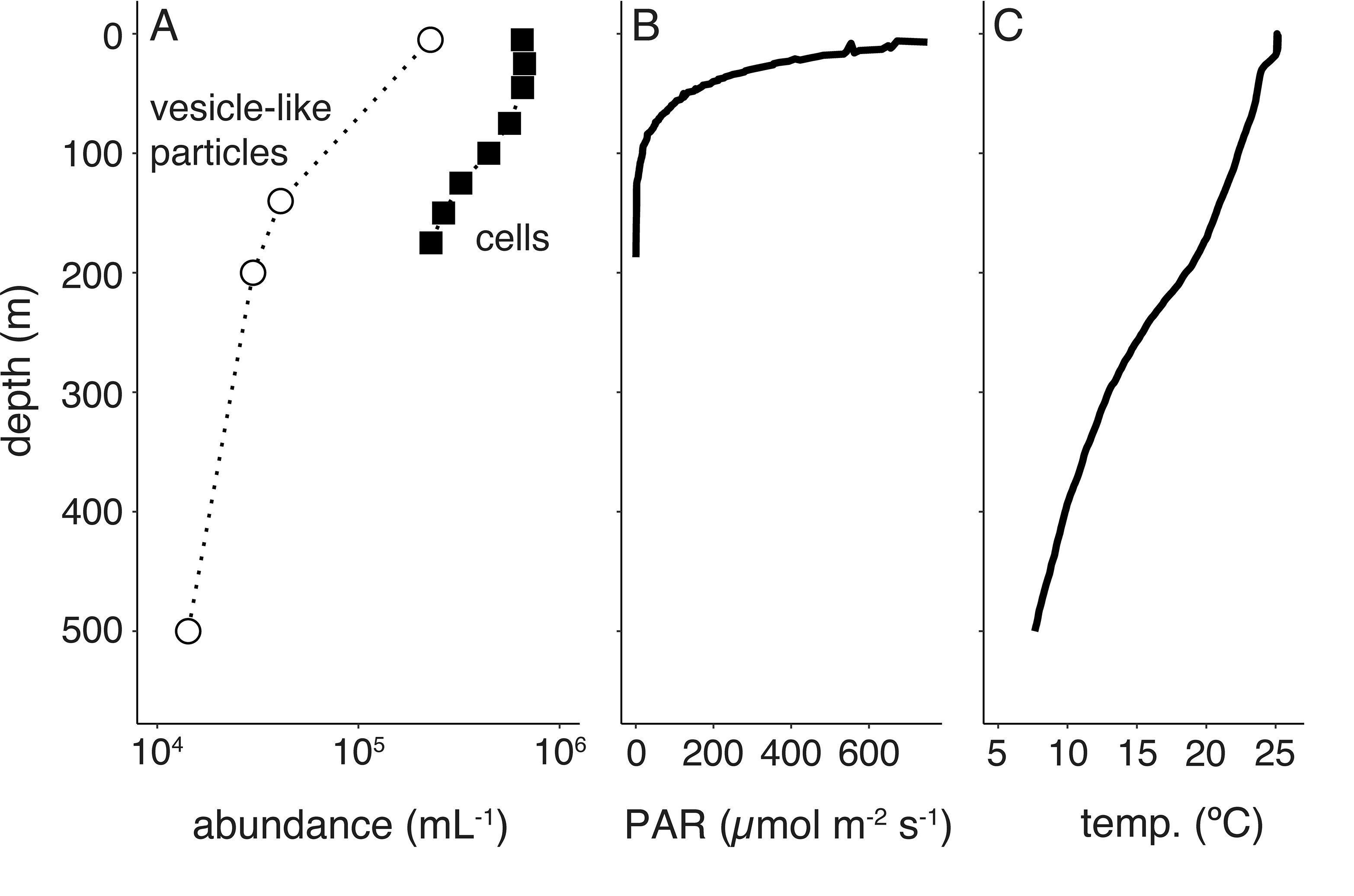 Environmental and taxonomic drivers of bacterial extracellular vesicle production in marine ecosystemsSteven J Biller, Allison Coe, Aldo A Arellano , and 6 more authorsApplied and Environmental Microbiology, Jun 2023
Environmental and taxonomic drivers of bacterial extracellular vesicle production in marine ecosystemsSteven J Biller, Allison Coe, Aldo A Arellano , and 6 more authorsApplied and Environmental Microbiology, Jun 2023Extracellular vesicles are small (approximately 50 to 250 nm in diameter), membrane-bound structures that are released by cells into their surrounding environment. Heterogeneous populations of vesicles are abundant in the global oceans, and they likely play a number of ecological roles in these microbially dominated ecosystems. Here, we examine how vesicle production and size vary among different strains of cultivated marine microbes as well as explore the degree to which this is influenced by key environmental variables. We show that both vesicle production rates and vesicle sizes significantly differ among cultures of marine Proteobacteria, Cyanobacteria, and Bacteroidetes. Further, these properties vary within individual strains as a function of differences in environmental conditions, such as nutrients, temperature, and light irradiance. Thus, both community composition and the local abiotic environment are expected to modulate the production and standing stock of vesicles in the oceans. Examining samples from the oligotrophic North Pacific Gyre, we show depth-dependent changes in the abundance of vesicle-like particles in the upper water column in a manner that is broadly consistent with culture observations: the highest vesicle abundances are found near the surface, where the light irradiances and the temperatures are the greatest, and they then decrease with depth. This work represents the beginnings of a quantitative framework for describing extracellular vesicle dynamics in the oceans, which is essential as we begin to incorporate vesicles into our ecological and biogeochemical understanding of marine ecosystems. Bacteria release extracellular vesicles that contain a wide variety of cellular compounds, including lipids, proteins, nucleic acids, and small molecules, into their surrounding environment. These structures are found in diverse microbial habitats, including the oceans, where their distributions vary throughout the water column and likely affect their functional impacts within microbial ecosystems. Using a quantitative analysis of marine microbial cultures, we show that bacterial vesicle production in the oceans is shaped by a combination of biotic and abiotic factors. Different marine taxa release vesicles at rates that vary across an order of magnitude, and vesicle production changes dynamically as a function of environmental conditions. These findings represent a step forward in our understanding of bacterial extracellular vesicle production dynamics and provide a basis for the quantitative exploration of the factors that shape vesicle dynamics in natural ecosystems.
-
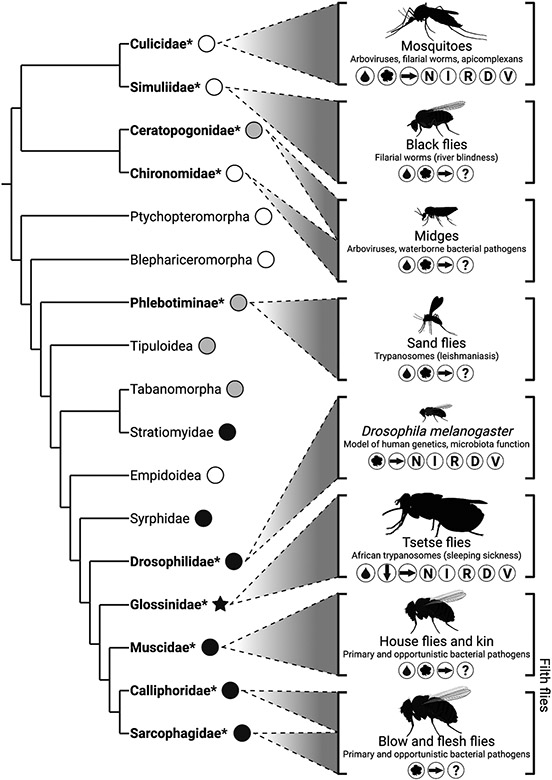 Beyond canonical models: why a broader understanding of Diptera-microbiota interactions is essential for vector-borne disease controlAldo A Arellano, Andrew J Sommer, and Kerri L CoonEvolutionary ecology, Feb 2023
Beyond canonical models: why a broader understanding of Diptera-microbiota interactions is essential for vector-borne disease controlAldo A Arellano, Andrew J Sommer, and Kerri L CoonEvolutionary ecology, Feb 2023Vector-borne diseases constitute a major global public health threat. The most significant arthropod disease vectors are predominantly comprised of members of the insect order Diptera (true flies), which have long been the focus of research into host-pathogen dynamics. Recent studies have revealed the underappreciated diversity and function of dipteran-associated gut microbial communities, with important implications for dipteran physiology, ecology, and pathogen transmission. However, the effective parameterization of these aspects into epidemiological models will require a comprehensive study of microbe-dipteran interactions across vectors and related species. Here, we synthesize recent research into microbial communities associated with major families of dipteran vectors and highlight the importance of development and expansion of experimentally tractable models across Diptera towards understanding the functional roles of the gut microbiota in modulating disease transmission. We then posit why further study of these and other dipteran insects is not only essential to a comprehensive understanding of how to integrate vector-microbiota interactions into existing epidemiological frameworks, but our understanding of the ecology and evolution of animal-microbe symbiosis more broadly.
-
 Novel integrative elements and genomic plasticity in ocean ecosystemsThomas Hackl, Raphaël Laurenceau, Markus J Ankenbrand , and 8 more authorsCell, Jan 2023
Novel integrative elements and genomic plasticity in ocean ecosystemsThomas Hackl, Raphaël Laurenceau, Markus J Ankenbrand , and 8 more authorsCell, Jan 2023Horizontal gene transfer accelerates microbial evolution. The marine picocyanobacterium Prochlorococcus exhibits high genomic plasticity, yet the underlying mechanisms are elusive. Here, we report a novel family of DNA transposons-"tycheposons"-some of which are viral satellites while others carry cargo, such as nutrient-acquisition genes, which shape the genetic variability in this globally abundant genus. Tycheposons share distinctive mobile-lifecycle-linked hallmark genes, including a deep-branching site-specific tyrosine recombinase. Their excision and integration at tRNA genes appear to drive the remodeling of genomic islands-key reservoirs for flexible genes in bacteria. In a selection experiment, tycheposons harboring a nitrate assimilation cassette were dynamically gained and lost, thereby promoting chromosomal rearrangements and host adaptation. Vesicles and phage particles harvested from seawater are enriched in tycheposons, providing a means for their dispersal in the wild. Similar elements are found in microbes co-occurring with Prochlorococcus, suggesting a common mechanism for microbial diversification in the vast oligotrophic oceans.
2022
-
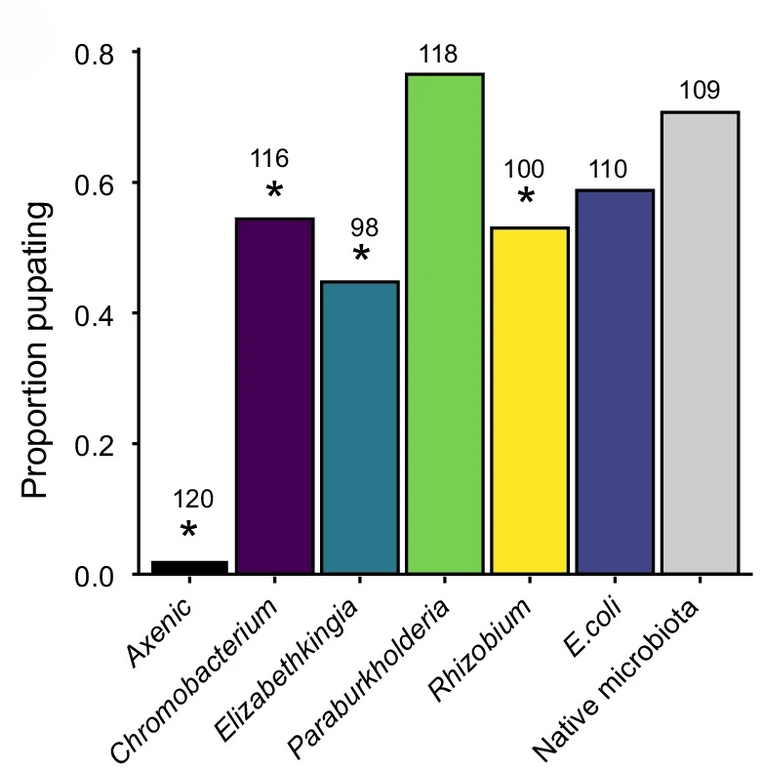 Bacterial communities in carnivorous pitcher plants colonize and persist in inquiline mosquitoesAldo A Arellano, and Kerri L CoonAnimal Microbiome, Feb 2022
Bacterial communities in carnivorous pitcher plants colonize and persist in inquiline mosquitoesAldo A Arellano, and Kerri L CoonAnimal Microbiome, Feb 2022The leaves of carnivorous pitcher plants harbor diverse communities of inquiline species, including bacteria and larvae of the pitcher plant mosquito (Wyeomyia smithii), which aid the plant by processing captured prey. Despite the growing appreciation for this microecosystem as a tractable model in which to study food web dynamics and the moniker of W. smithii as a ‘keystone predator’, very little is known about microbiota acquisition and assembly in W. smithii mosquitoes or the impacts of W. smithii-microbiota interactions on mosquito and/or plant fitness. In this study, we used high throughput sequencing of bacterial 16S rRNA gene amplicons to characterize and compare microbiota diversity in field- and laboratory-derived W. smithii larvae. We then conducted controlled experiments in the laboratory to better understand the factors shaping microbiota acquisition and persistence across the W. smithii life cycle. Methods were also developed to produce axenic (microbiota-free) W. smithii larvae that can be selectively recolonized with one or more known bacterial species in order to study microbiota function. Our results support a dominant role for the pitcher environment in shaping microbiota diversity in W. smithii larvae, while also indicating that pitcher-associated microbiota can persist in and be dispersed by adult W. smithii mosquitoes. We also demonstrate the successful generation of axenic W. smithii larvae and report variable fitness outcomes in gnotobiotic larvae monocolonized by individual bacterial isolates derived from naturally occurring pitchers in the field. This study provides the first information on microbiota acquisition and assembly in W. smithii mosquitoes. This study also provides the first evidence for successful microbiota manipulation in this species. Altogether, our results highlight the value of such methods for studying host-microbiota interactions and lay the foundation for future studies to understand how W. smithii-microbiota interactions shape the structure and stability of this important model ecosystem.
-
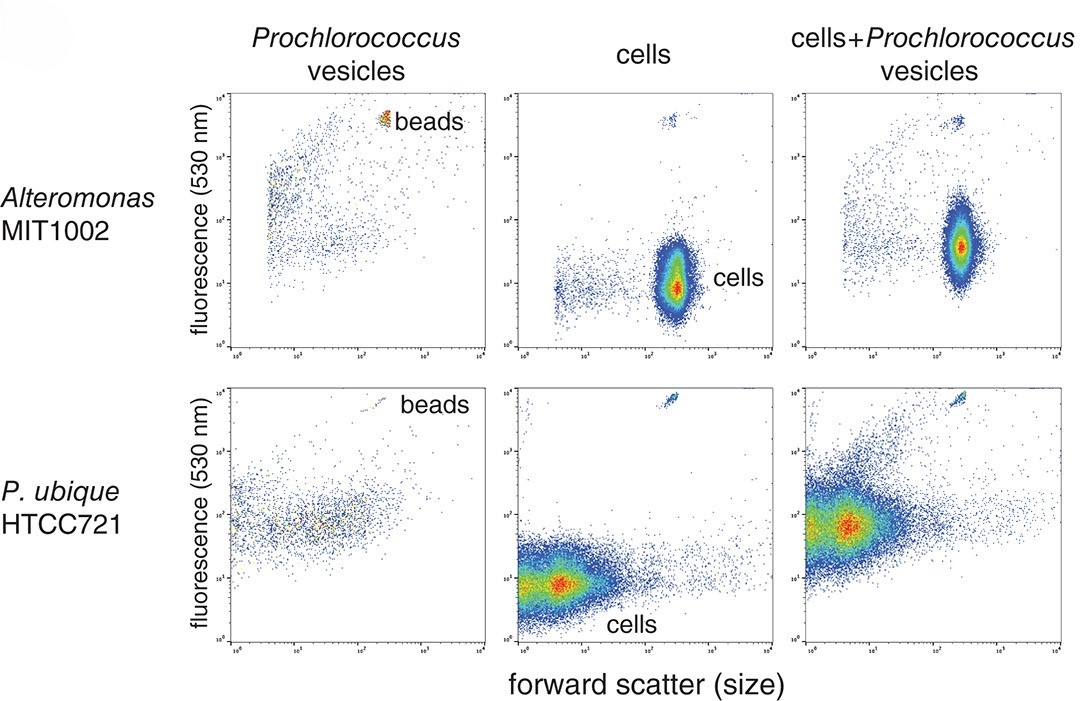 Prochlorococcus extracellular vesicles: molecular composition and adsorption to diverse microbesSteven J Biller, Rachel A Lundeen, Laura R Hmelo , and 8 more authorsEnvironmental Microbiology, Jan 2022
Prochlorococcus extracellular vesicles: molecular composition and adsorption to diverse microbesSteven J Biller, Rachel A Lundeen, Laura R Hmelo , and 8 more authorsEnvironmental Microbiology, Jan 2022Extracellular vesicles are small ( 50–200 nm diameter) membrane-bound structures released by cells from all domains of life. While vesicles are abundant in the oceans, their functions, both for cells themselves and the emergent ecosystem, remain a mystery. To better characterize these particles – a prerequisite for determining function – we analysed the lipid, protein, and metabolite content of vesicles produced by the marine cyanobacterium Prochlorococcus. We show that Prochlorococcus exports a diverse array of cellular compounds into the surrounding seawater enclosed within discrete vesicles. Vesicles produced by two different strains contain some materials in common, but also display numerous strain-specific differences, reflecting functional complexity within vesicle populations. The vesicles contain active enzymes, indicating that they can mediate extracellular biogeochemical reactions in the ocean. We further demonstrate that vesicles from Prochlorococcus and other bacteria associate with diverse microbes including the most abundant marine bacterium, Pelagibacter. Together, our data point toward hypotheses concerning the functional roles of vesicles in marine ecosystems including, but not limited to, possibly mediating energy and nutrient transfers, catalysing extracellular biochemical reactions, and mitigating toxicity of reactive oxygen species.
2021
-
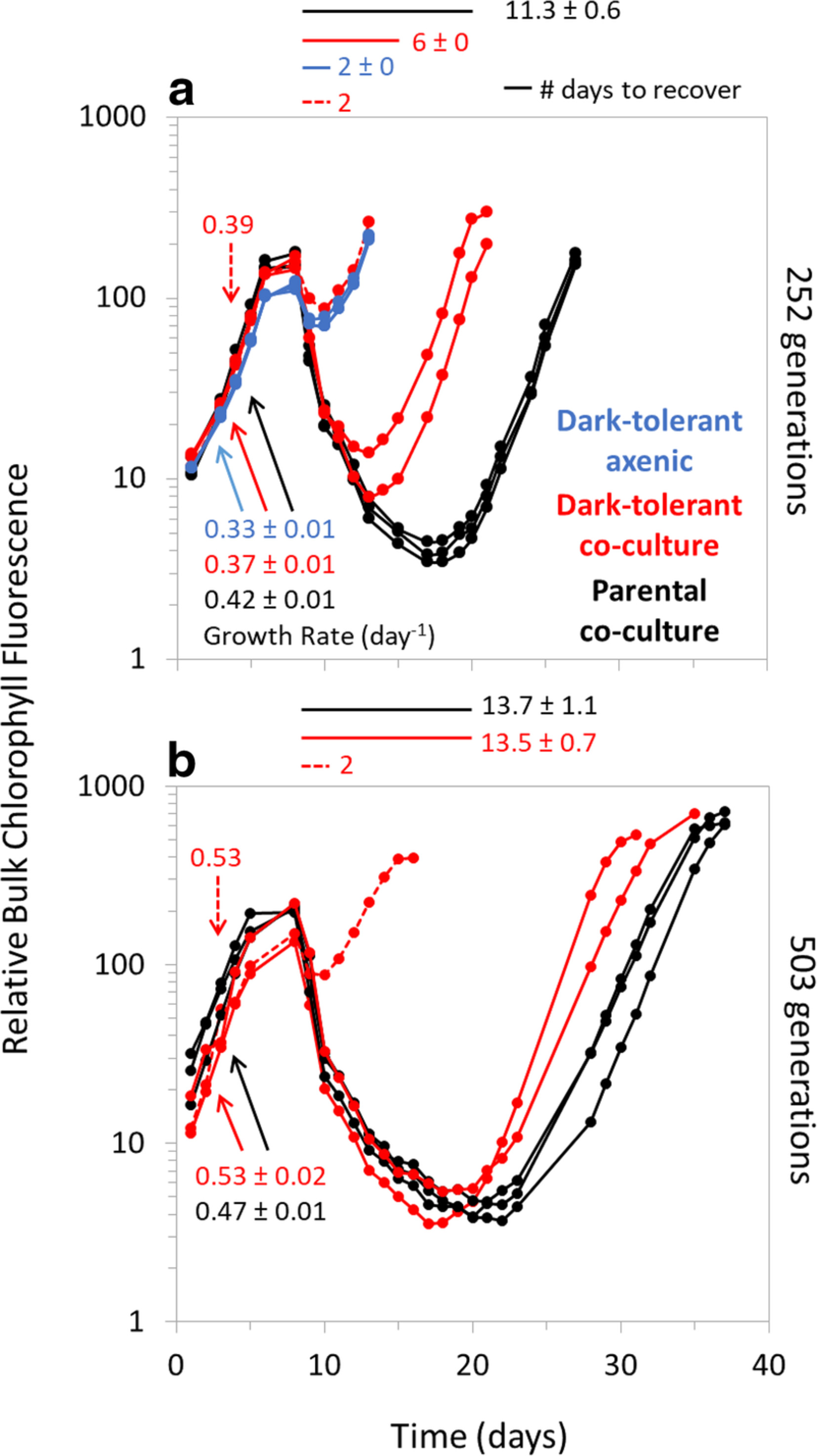 Coping with darkness: The adaptive response of marine picocyanobacteria to repeated light energy deprivationAllison Coe, Steven J Biller, Elaina Thomas , and 8 more authorsLimnology and oceanography, Sep 2021
Coping with darkness: The adaptive response of marine picocyanobacteria to repeated light energy deprivationAllison Coe, Steven J Biller, Elaina Thomas , and 8 more authorsLimnology and oceanography, Sep 2021The picocyanobacteria Prochlorococcus and Synechococcus are found throughout the ocean’s euphotic zone, where the daily light:dark cycle drives their physiology. Periodic deep mixing events can, however, move cells below this region, depriving them of light for extended periods of time. Here, we demonstrate that members of these genera can adapt to tolerate repeated periods of light energy deprivation. Strains kept in the dark for 3 d and then returned to the light initially required 18–26 d to resume growth, but after multiple rounds of dark exposure they began to regrow after only 1–2 d. This dark-tolerant phenotype was stable and heritable; some cultures retained the trait for over 132 generations even when grown in a standard 13:11 light:dark cycle. We found no genetic differences between the dark-tolerant and parental strains of Prochlorococcus NATL2A, indicating that an epigenetic change is likely responsible for the adaptation. To begin to explore this possibility, we asked whether DNA methylation—one potential mechanism mediating epigenetic inheritance in bacteria—occurs in Prochlorococcus. LC–MS/MS analysis showed that while DNA methylations, including 6 mA and 5 mC, are found in some other Prochlorococcus strains, there were no methylations detected in either the parental or dark-tolerant NATL2A strains. These findings suggest that Prochlorococcus utilizes a yet-to-be-determined epigenetic mechanism to adapt to the stress of extended light energy deprivation, and highlights phenotypic heterogeneity as an additional dimension of Prochlorococcus diversity.
2020
-
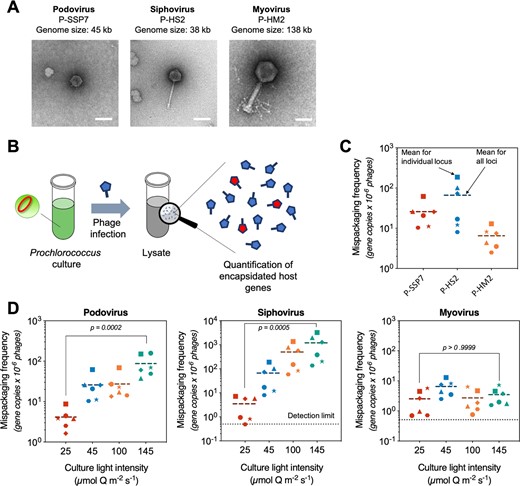 Frequency of mispackaging of Prochlorococcus DNA by cyanophageRaphaël Laurenceau, Nicolas Raho, Mathieu Forget , and 2 more authorsThe ISME Journal, Sep 2020
Frequency of mispackaging of Prochlorococcus DNA by cyanophageRaphaël Laurenceau, Nicolas Raho, Mathieu Forget , and 2 more authorsThe ISME Journal, Sep 2020Prochlorococcus cells are the numerically dominant phototrophs in the open ocean. Cyanophages that infect them are a notable fraction of the total viral population in the euphotic zone, and, as vehicles of horizontal gene transfer, appear to drive their evolution. Here we examine the propensity of three cyanophages—a podovirus, a siphovirus, and a myovirus—to mispackage host DNA in their capsids while infecting Prochlorococcus, the first step in phage-mediated horizontal gene transfer. We find the mispackaging frequencies are distinctly different among the three phages. Myoviruses mispackage host DNA at low and seemingly fixed frequencies, while podo- and siphoviruses vary in their mispackaging frequencies by orders of magnitude depending on growth light intensity. We link this difference to the concentration of intracellular reactive oxygen species and protein synthesis rates, both parameters increasing in response to higher light intensity. Based on our findings, we propose a model of mispackaging frequency determined by the imbalance between the production of capsids and the number of phage genome copies during infection: when protein synthesis rate increase to levels that the phage cannot regulate, they lead to an accumulation of empty capsids, in turn triggering more frequent host DNA mispackaging errors.
2018
-
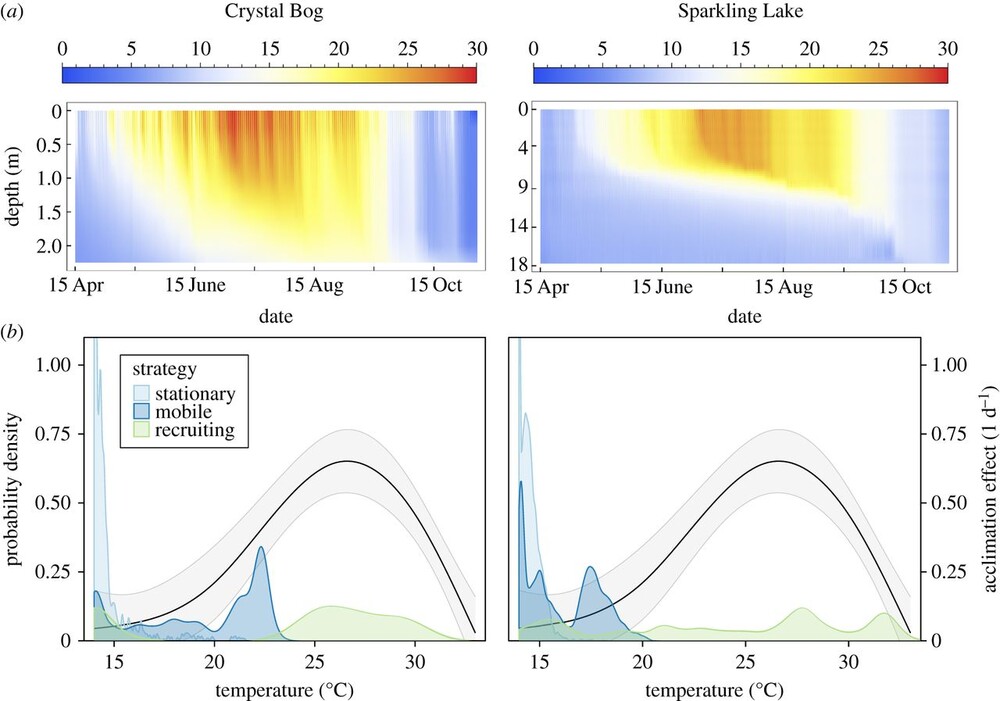 Gradual plasticity alters population dynamics in variable environments: thermal acclimation in the green alga Chlamydomonas reinhartdiiColin T Kremer, Samuel B Fey, Aldo A Arellano , and 1 more authorProceedings of the Royal Society B: Biological Sciences, Jan 2018
Gradual plasticity alters population dynamics in variable environments: thermal acclimation in the green alga Chlamydomonas reinhartdiiColin T Kremer, Samuel B Fey, Aldo A Arellano , and 1 more authorProceedings of the Royal Society B: Biological Sciences, Jan 2018Environmental variability is ubiquitous, but its effects on populations are not fully understood or predictable. Recent attention has focused on how rapid evolution can impact ecological dynamics via adaptive trait change. However, the impact of trait change arising from plastic responses has received less attention, and is often assumed to optimize performance and unfold on a separate, faster timescale than ecological dynamics. Challenging these assumptions, we propose that gradual plasticity is important for ecological dynamics, and present a study of the plastic responses of the freshwater green algae Chlamydomonas reinhardtii as it acclimates to temperature changes. First, we show that C. reinhardtii’s gradual acclimation responses can both enhance and suppress its performance after a perturbation, depending on its prior thermal history. Second, we demonstrate that where conventional approaches fail to predict the population dynamics of C. reinhardtii exposed to temperature fluctuations, a new model of gradual acclimation succeeds. Finally, using high-resolution data, we show that phytoplankton in lake ecosystems can experience thermal variation sufficient to make acclimation relevant. These results challenge prevailing assumptions about plasticity’s interactions with ecological dynamics. Amidst the current emphasis on rapid evolution, it is critical that we also develop predictive methods accounting for plasticity.